Technology
We make precision medicines leveraging our proven platforms
Our proprietary CRISPR and delivery platforms allows us to specifically target and remove any antimicrobial resistance gene or kill any bacterium. Our technology is agnostic to any antimicrobial resistance profile of the target bacteria. It all starts with programming our CRISPR systems
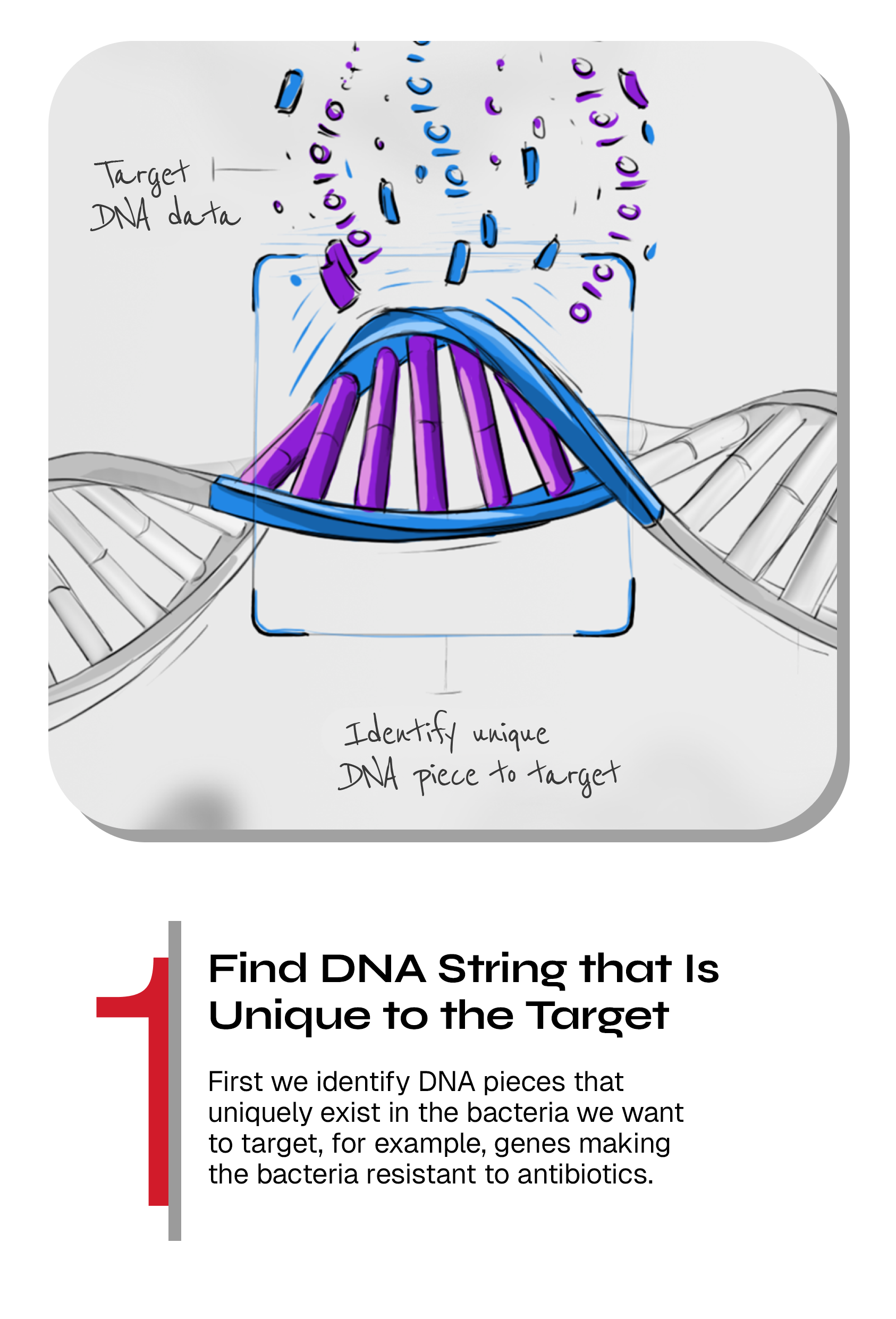
We program CRISPR to specifically target genes in bacteria of our choice
CRISPR is short for Clustered Regularly Interspaced Short Palindromic Repeats. CRISPR is naturally occurring as defense systems in bacteria and have revolutionized synthetic biology with the ability to program CRISPR to precisely recognize and cut DNA sequences of choice – providing scientists with a very precise molecular scalpel.
At SNIPR, we use CRISPR to find, cut and remove genes or bacteria of our choice. Each human being have around 30 trillion healthy bacteria living in our bodies, so we need to make precision medicines that will only kill the harmful bacteria. The vast amount of available genomic data from our internal and public databases allow our bioinformaticians to determine unique genetic fingerprints in bacteria that we want to kill that are not present in bacteria we want to keep.
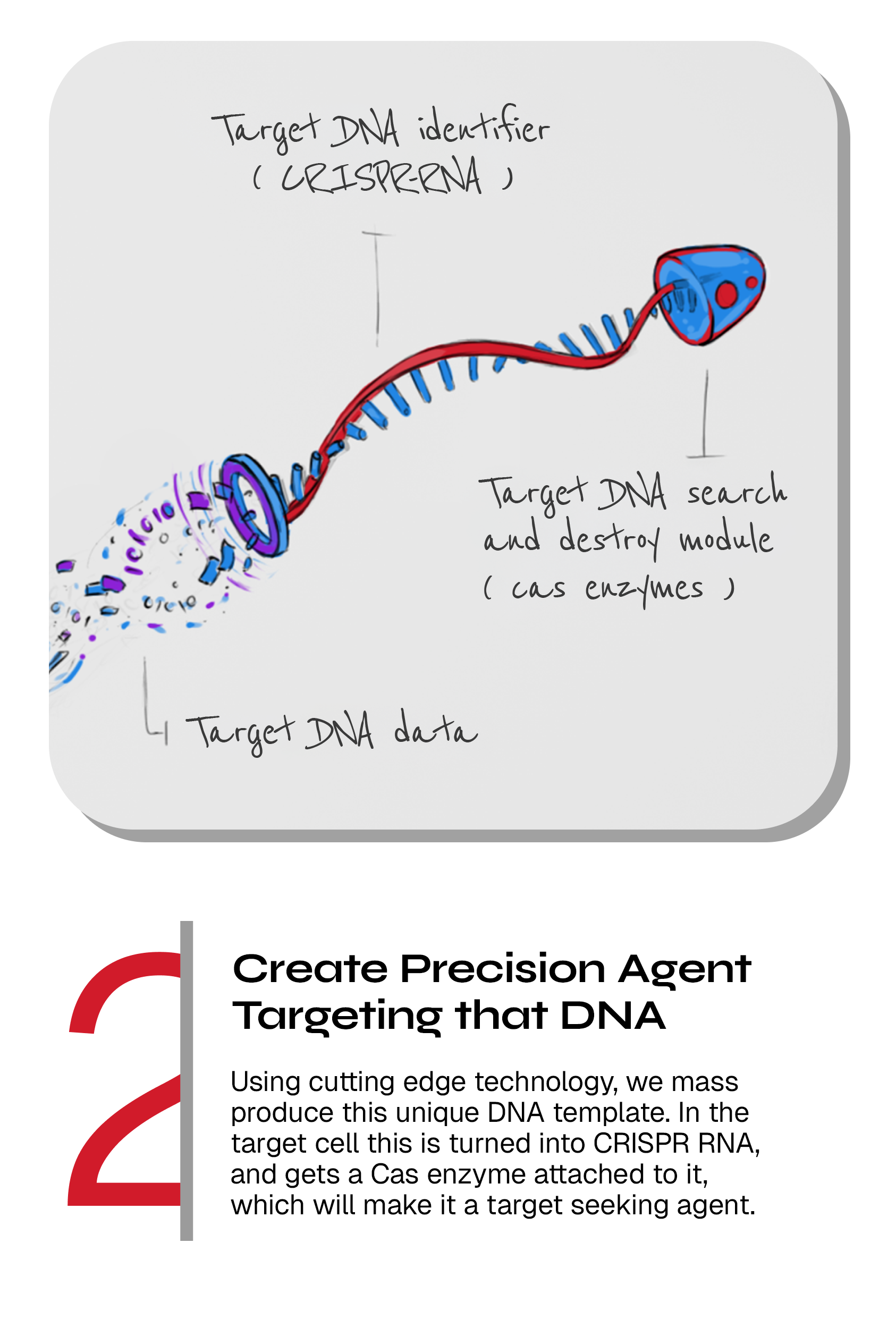
Engineering the precision targeting CRISPR agent
When the unique DNA sequences have been determined, they are transformed into so-called spacer sequences that our engineers assemble into CRISPR arrays.
SNIPR’s CRISPR-guided vectors are based on DNA, which encodes CRISPR and associated Cas enzymes. Upon delivery to the target bacterial cell, the CRISPR arrays and Cas enzymes are produced and assemble into the CRISPR-RNA/Cas complex needed for the programmed cutting of DNA of our choice. The arrays function as guides when our CRISPR-Cas systems search and destroy those unique sequences in the target bacteria.
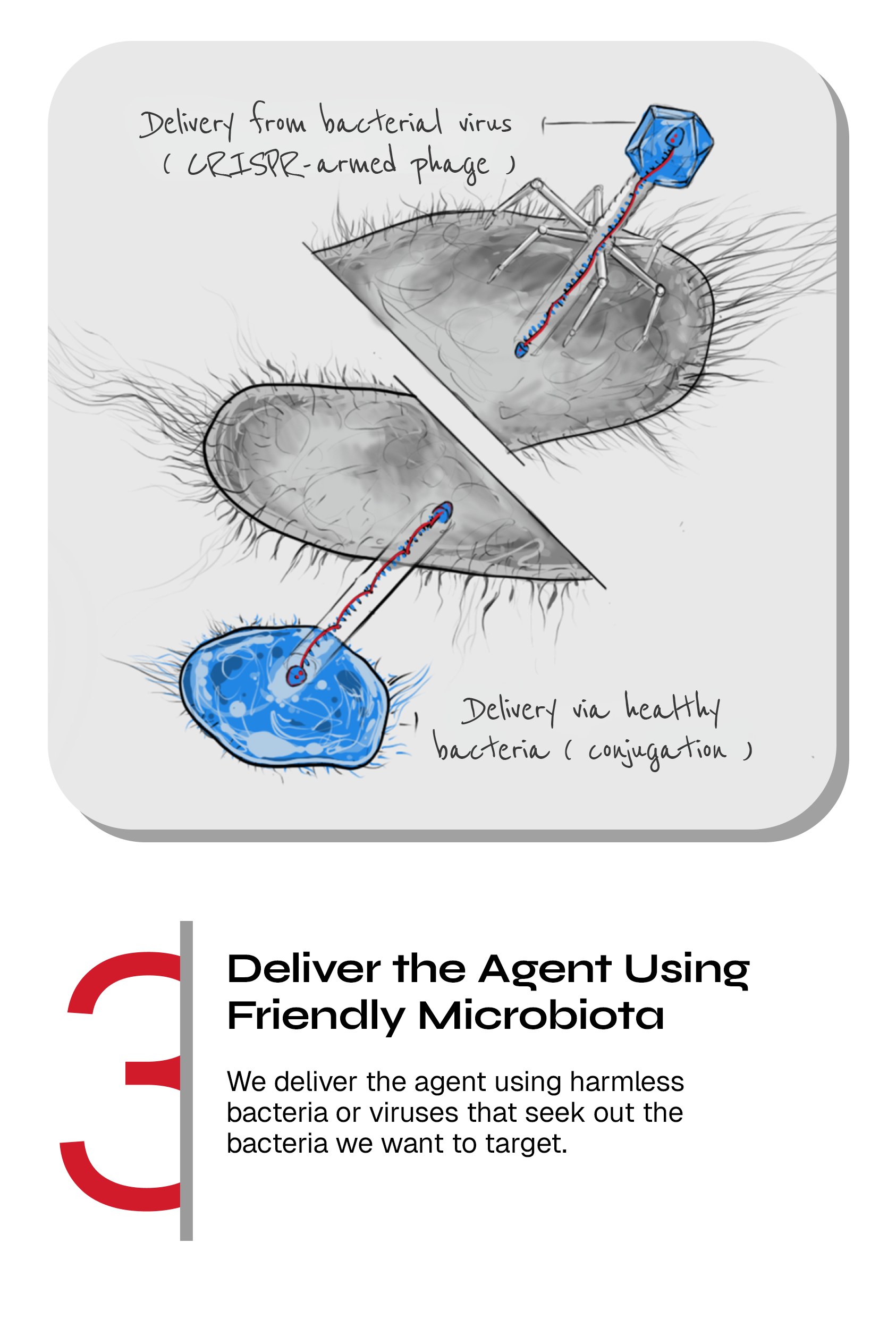
Bacterial viruses (phages) or harmless bacteria can be armed with CRISPR to become efficient delivery vehicles
CRISPR-armed phages: We incorporate our CRISPR-guided vectors comprising the CRISPR-Cas systems into the genome of bacteriophages (phages are viruses that only kill bacteria) to use them as CRISPR-delivery vehicles. Phages are everywhere outside and inside your body and are completely harmless to humans.
Phages are natural killers of bacteria and we make them even better by engineering.
Firstly, for a phage to infect a bacterial cell, it needs to bind surface structures using the tail-fibers. SNIPR is world leading in tail-fiber engineering. By using our deep knowledge of the phage adsorption biology as well as rational design of tail-fibers and combination of phages, we ensure that our phages robustly infect target bacteria of our choice.
Upon successful adsorption the CRISPR-armed phage DNA is injected into the target cell, where the CRISPR-Cas systems are turned into the programmed search and destroy molecules.
CRISPR-armed bacteria: The majority of bacteria living in and on your body are good and health-promoting and it is common to use probiotics (formulated good bacteria) to obtain various health benefits.
We have developed a parallel CRISPR delivery platform where we use harmless bacteria to combat harmful bacteria. We arm the harmless bacteria with CRISPR-Cas that they can transfer to neighboring harmful bacteria in their environment through a “tube”. This transfer process is called bacterial conjugation.
Conjugation is a natural mechanism used by bacteria for gene-sharing between each other. This is a way for bacteria to obtain beneficial genes and conjugation is therefore widespread among different bacteria.
We hijack this mechanism and use the widespread gene sharing to our benefit to broadly delivery our CRISPR-Cas systems from a harmless bacterial chassis strain to harmful target bacteria.
Our CRISPR-armed phage platform was developed for SNIPR001 – a cocktail of CRISPR-armed phages targeting E. coli (currently in Phase 2 clinical trials). The development of SNIPR001 was published in Nature Biotech and the Phase 1 trial in Lancet Microbe. The CAP platform tech is now being expanded to target Klebsiella pneumoniae and E.coli in Environmental Enteric Disease with The Gates Foundation and in Pseudomonas aeruginosa with Cystic Fibrosis Foundation. Our CRISPR-armed bacteria platform is currently being used to resensitize the world’s most dangerous antimicrobial resistant pathogens to the already existing safe and affordable antibiotics together with SPRIN-D.
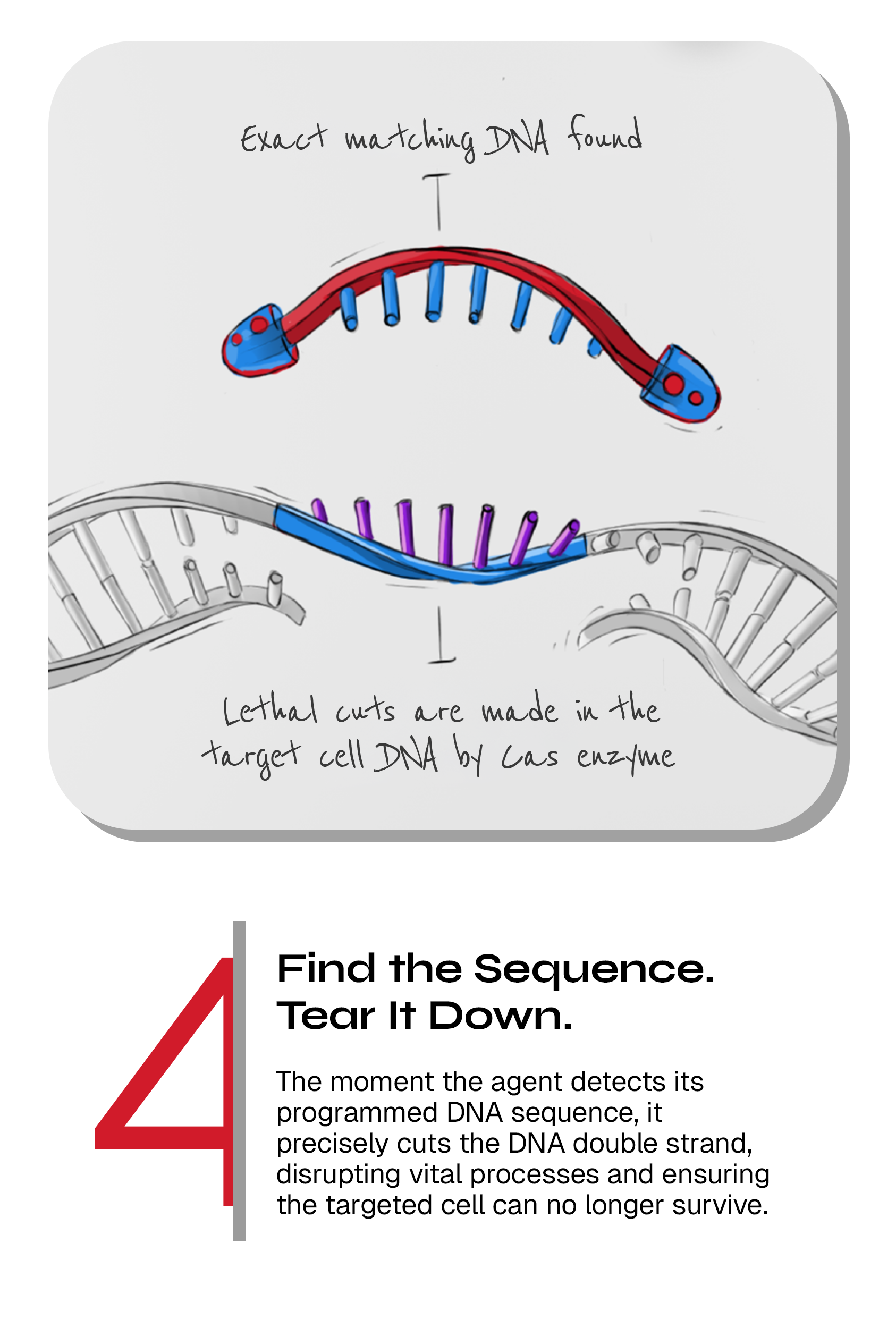
Programmed CRISPR arrays and Cas enzymes work together to find and cut selected genes in target bacteria
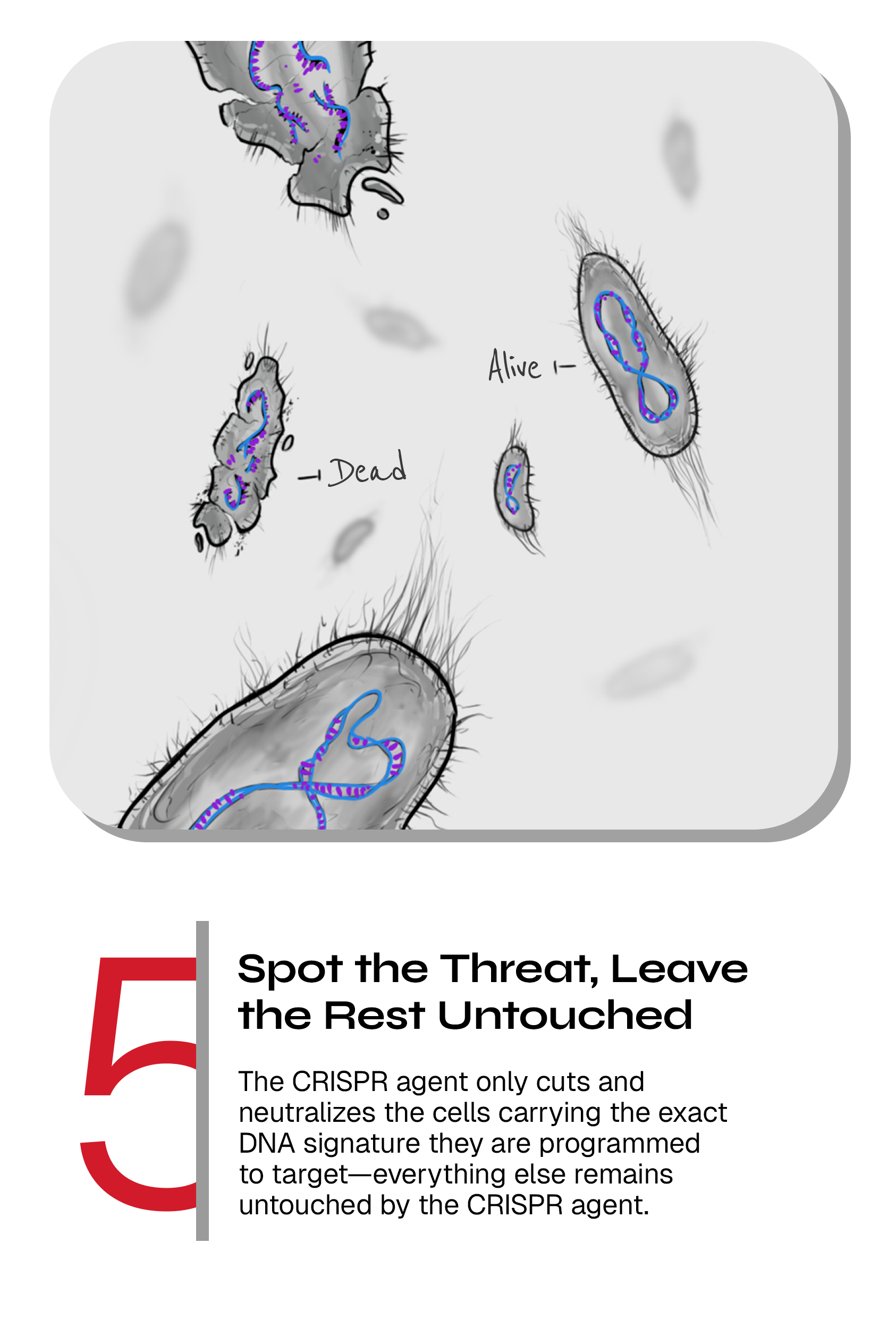
Upon delivery to the target cell, the activated CRISPR-Cas complex is patrolling the bacterial cell, searching for the matching sequence that we have programmed it to cut. If no such sequence is present in the bacterial cell (e.g. if the bacterium is belonging to the healthy group), the system does not harm the cell but if the CRISPR-Cas complex find a matching sequence (if the bacterium is a harmful target bacterium), the Cas enzymes will efficiently cut the DNA, which in turn leads to specific killing of that cell. This way, we routinely kill >99.9% of the targeted bacteria. The CRISPR-Cas systems are also designed to be active and kill bacteria that are not growing – a state in which antibiotics are not functional.
Killing the bad bacteria while keeping the good bacteria alive
Our precision-killing CRISPR technology allows us to program the CRISPR-guided vectors to only targeting the bacteria of our choice. The end result is therefore precision killing of the harmful bacteria, that we have programmed the CRISPR-guided vector to target, while leaving the good bacteria intact and hence preserving the microbiome.
When we use the CRISPR-Cas systems to potentiate our phage platform, we get a further layer of killing determined by the phage natural lytic activity. The phage’s natural killing activity is in itself very specific so the combination with CRISPR results in a potent dual-function precision kill modality.
Healthy bacteria can be engineered to be local “inner pharmacies” in the gut
In some specific cases, typically related to cardiometabolic disease or inflammation & immunology, we have developed our bacterial chassis strain to function as a producer of wanted therapeutics locally in the gut. This has benefits of being able to produce molecules where they are needed at low cost. Here, we have two platforms 1) conversion and 2) expression, both based on the same bacterial chassis strain.
In the conversion platform, the good bacterium takes up one (typically unwanted) metabolite and converts it into a health-promoting metabolite. Using this unique chassis strain and our extensive engineering capabilities, we have generated good bacteria, which in animals have shown to have health promoting effects in different models of T2D, colitis and obesity.
The expression platform leverages the same acterial chassis but here the engineering is focused on secreting molecules such as nanobodies. We have preclinical proof in Parkinson’s disease.